KEY TO CHAPTER 9 EXERCISES
(Susan Odom and Arthur Cammers, 2019)
Exercise 9.2.1 In Example 9.1.1, note that DMSO is not written into the rate equation, yet if we raise or lower the volume of DMSO, the rate of the reaction changes. Provide an explanation for this phenomenon.
Answer: DMSO
is not involved in the rate determining step in that it is not part of the bond
cleavage or formation involved, but the volume of DMSO affects the
concentration of propanol and sodium hydroxide. So, if you keep the mass of
reagents the same and decrease the volume of the solvent DMSO, the reaction
rate will increase. Vice versa, increasing solvent volume will lower the
concentration of the reagents and slow the reaction rate.
Exercise 9.2.2 In the reaction below, the reaction rate is dependent only on the concentration of tert-butanol and can be written as rate = k[t-BuOH]. What does this tell you about the involvement of HCl in the rate determining step?
![]()
Answer: By
HCl not being involved in the rate determining step, this tells you only one
species is involved. That said, HCl is involved in this reaction, as first an
acid-base reaction with HCl occurs – it’s just not the slow step, as acid-base
reactions are quite fast compared to others.
Exercise 9.3.1 What is the measure in degrees) of the H-C-O angle in the SN2 transition state illustrated above?
Reminder of transition state:
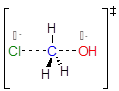
Answer: 90º The C─H bonds are hybridized sp2. In the transition state the Cl—C—OH connection is composed of: 1) an sp hybrid orbital at Cl, 2) an sp hybrid orbital at OH, and 3) a pure p orbital at C.
Exercise 9.3.2 Draw a mechanism for the SN1 solvolysis of tert-butyl chloride in methanol. What new functional group has been formed?
Answer:
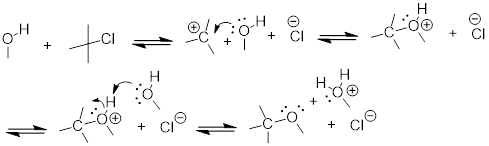
The organic product is an ether, t-butyl methyl ether: CH3)3COCH3
Exercise
9.3.3
a) Draw a complete mechanism for the hydrolysis reaction in the previous figure, showing all bond-breaking and bond-forming steps, and all intermediate species.
b) Draw structures representing TS1 and TS2 in the reaction. Use the solid/dash wedge convention to show three dimensions.
c) What is the expected optical rotation of the product mixture?
d) Could the two organic products be separated on a silica column chromatography?
Reminder of the reaction:
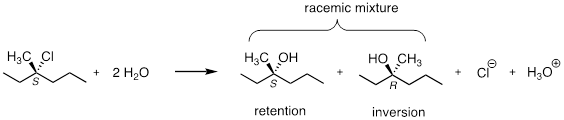
Answer:
a) See the mechanism in the previous exercise. The carbocation is planar, meaning that the C─O bond formation in the next step can occur from both side equally because these two processes are enantiomers of one another. The rate and equilibria of enantiomeric processes are equivalent because due to the fact that enantiomers have identical physico-chemical properties. The S and R alcohols above must be produced in a 1: 1 ratio.
b) These TS1 defines C─Cl bond breakage. TS2 involves C─O bond formation (two enantiomers).
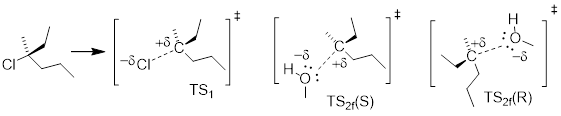
c) There will be no optical rotation.
d) No.
Exercise
9.3.4
a) Draw the product(s) of the hydrolysis of (R)-3-chloro-3-methylheptane.
b) What can you predict, if anything, about the optical rotation of the product(s)?
c) Draw the product(s) of the hydrolysis of (3R,5R)-3-chloro-3,5-dimethylheptane.
d) What can you predict, if anything, about the optical rotation of the product(s)?
Answer:
a) ![]()
b) There will be no optical rotation.
c) ![]()
d) There will be an optical rotation from the presence of two diastereomers.
Exercise 9.4.1 Which amino acid has the more nucleophilic side chain - serine or tyrosine? Explain.
Answer: Serine will have the more nucleophilic side chain because the alcohol is on an sp3 hyridized O atom, which has higher energy lone pairs than the lone pairs in tyrosine – one of which is in a p orbital and delocalized into the pi system, the other being in a sp2 orbital.
Exercise 9.4.2 In each pair, which is the better nucleophile: (a) A cysteine side chain or a methionine side chain? (b) A serine or a threonine? Explain.
Answer: (a) Cysteine, because the S in a thiol (RSH) is less hindered than the thioether (RSR’) in methionine. (b) Serine will have the more nucleophilic side chain because the alcohol is on a 1º C atom and is less hindered than the alcohol on the 2º C atom in threonine.
Exercise 9.4.3 In each of the following pairs of molecules/ions, which is expected to
react more rapidly with CH3Cl in acetone solvent? Explain your choice.
a) phenolate (deprotonated phenol) or benzoate (deprotonated benzoic acid)?
b) water or hydronium ion?
c) trimethylamine or triethylamine?
d) chloride anion or iodide anion?
e) CH3NH– or CH3CH2NH2?
f) acetate or trichloroacetate?
g) aniline or 4-methoxyaniline?
h) phenolate or 2,6-dimethylphenolate?
Answer:
a) Phenolate will react faster. The pKa of phenol is higher than benzoic acid. The O-atom as a nucleophile should be more reactive.
b) Water will react faster. The O-atom as a nucleophile should be more reactive in water hydronium ion is its conjugate acid. Hydronium ion IS the product of water and an electrophile: (H+).
c) The reactivity of trimethylamine and triethylamine will be determined by the steric difference between CH3 and CH2CH3. While previous discussions of A-values showed us that there is not a large size difference between CH3 and CH2CH3 here the net effect must be the sum of all three. Since the SN2 mechanism requires approach to within bond distances, the effect is noteworthy.
d) Chloride anion or iodide anion? This is a complicated issue, but one you should be aware of in your study of organic chemistry. https://www.name-reaction.com/finkelstein-reaction
e) CH3NH– or CH3CH2NH2? See answer for b)
f) acetate or trichloroacetate? See answer for a). This is the inductive effect through σ bonds of electronegative Cl.
g) aniline or 4-methoxyaniline? The 4-methoxy is electron donating, which increases the pKa of its basicity (decreases the acidity of its conj. acid relative to the conj. acid of aniline. See answer for a). If you didn’t know the structure of aniline you should have googled it and applied what you know about the reactivity of electrons to the structure.
h) See answer for g) The 2,6-dimethylphenolate will be more reactive due to the electron donation of the methoxy groups.
Exercise 9.5.1 Which would be expected to react more rapidly in an SN2 reaction with an azide ion (N3–) nucleophile in acetone solvent: 1-bromo-2,2-dimethylbutane or 1-bromo-3-methylbutane?
Answer: Since the SN2 mechanism requires approach to within bond distances the steric effect of the more proximal dimethyl substituents in 1-bromo-2,2-dimethylbutane will decelerate the reaction at the 2° C atom more than the methyl groups in 1-bromo-3-methylbutane decelerates the SN2 reaction at the 2° C atom.
Exercise 9.5.2 Fill in the missing numbers in this statement: The conjugated π system in the benzylic carbocation above is composed of ___7___ p orbitals overlapping to share __6____ p electrons.
Exercise 9.5.3 rank the following carbocations from most to least stable:
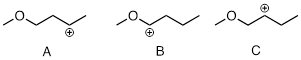
Answer: B is most stable due to π bonding, C is probably least stable due to bond-number proximity to the O atom which draws electron density due to its electronegativity.
Exercise 9.5.4 Explain why vinylic carbocations are unstable. (Hint: think about hybridization and electronegativity)
Answer: The p orbital of the linear vinylic carbocation is not very stabilized by hyperconjugation.
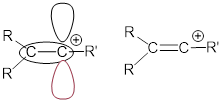
There is only one group that can stabilize the C center: R’ in the figure above. Bond formation at the C atom should be highly enthalpically favored because an sp2 bond at the C atom forms straight away from the poorly stabilized p orbital of the cation.
Exercise 9.5.5 The carbocation below is an intermediate species in a reaction that is part of the biosynthesis of a hallucinogenic compound in a fungus. Draw a resonance contributor that shows how it is stabilized by resonance with the nitrogen atom.
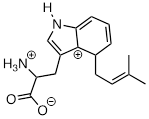
Answer:
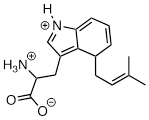
Exercise
9.5.6
Draw a resonance structure of the crystal violet cation in which the positive charge is delocalized to one of the nitrogen atoms.
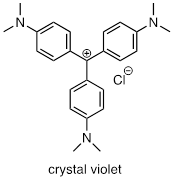
b) Notice that crystal violet is deeply colored. Explain why you could have predicted this from looking at its chemical structure.
c) The conjugated system of crystal violet consists of how many overlapping p orbitals sharing how many p electrons?
Answer: a) See previous answer
b) The cation is composed of 22 conjugated p orbitals. The effect of conjugation decreases the difference in energy between the HOMO and LUMO. See sections on UV-Vis spectroscopy.
c) … see b)
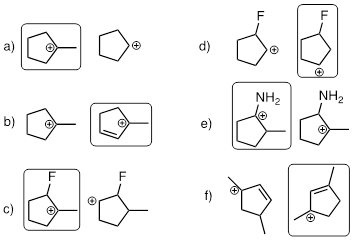
Exercise 9.5.7 State which carbocation in each pair below is more stable, or if they are expected to be approximately equal.

Answer:
a) First cation is more stable because it is more substituted.
b) Second cation is more stable because it is conjugated.
c) First cation is more stable because it is more substituted.
d) First cation is more stable because it is farther in terms of bonds away from the electronegative F atom.
e) First cation is more stable because it is conjugated by the N atom lone pair.
f) Second cation is more stable because it is equally conjugated but is more substituted.
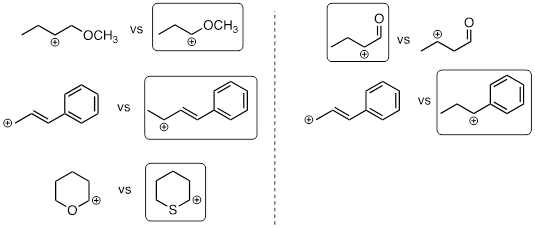
Exercise 9.6.1 In each pair (A and B) below, which electrophile would be expected to react more rapidly with cyanide ion nucleophile in acetone solvent?
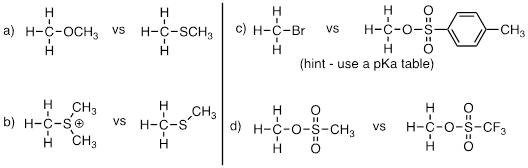
Answer:

Exercise 9.9.1 Think back to the acid-base chapter: the pKa of a protonated ether is approximately zero, indicating that an ether is a very weak base. Considering periodic trends in acidity and basicity, what can you say about the relative basicity of a sulfide?
Answer: The pKa of Me2OH(+) = -6.5 whereas the the pKa of Me2SH(+) = -5.4 Ref: https://www.chem.wisc.edu/areas/reich/pkatable/ This means that once released the lone pair on the O atom is more stable than the lone pair on the S atom in their respective conjugate bases which points to slightly more basicity of the thioether than the ether. Remember that increasing electronegativity as you go up the column in the periodic table decreases the basicity.
Exercise 9.9.2 SAM is formed by a nucleophilic substitution reaction between methionine and adenosine triphosphate (ATP). Draw a mechanism for this reaction, and explain why you chose either an SN1 or and SN2 pathway.
Answer: See section9.10.1. This is an enzyme-catalyzed reaction. The triphosphate group is promoted to leave by the catalytic mechanism. An enzyme-bound cation could be a catalytic intermediate (more like SN1) or the triphosphate could leave in a concerted fashion to the C─S bond formation (SN2). This reaction cannot be true SN1 because ATP is stable to water and becomes activated in the enzyme context and because departure of the triphosphate anion would leave a 1° carbocation.
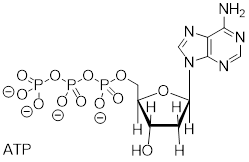
ATP + methionine adenosyltransferase + methionine ─► SAM
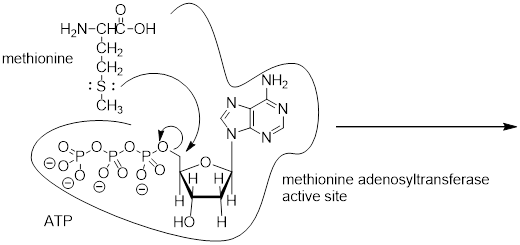
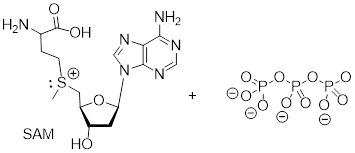
Exercise 9.10.1 A rookie organic chemist ran the reaction shown above, hoping to synthesize an ether. Instead, he got the alkene shown above. What alkyl halide/alcohol combination should he have used instead to get the ether product he was trying for?
The reaction:
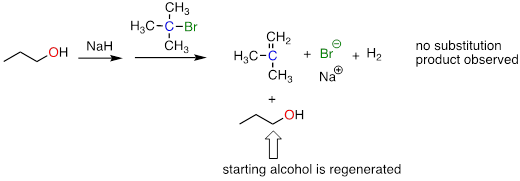
Answer:
![]()
KEY TO CHAPTER 9 PRACTICE PROBLEMS
PP 9-01 Answer: D > B > A > C
PP 9-02 Draw line structures representing the most stable cation with the given molecular formula:
a) C3H7+ b) C4H9+ c) C3H8N+ d) C4H7+
Answer:
When going from formula to structure DU is always the first step.
Think about
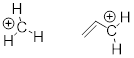
Add H:(-)

DU = 0 and 1 respectively .
For a) C3H7++ H:(-) ─► C3H8
b) C4H9++ H:(-) ─► C4H10 DU = 0
c) C3H8N++ H:(-) ─► C3H9N DU = 0
d) C4H7++ H:(-) ─► C4H8 DU = 1
a) ![]()
b) ![]()
c) 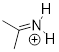 most stable resonance
representation of
most stable resonance
representation of 
d)  subtract H:(-) from
subtract H:(-) from ![]() or
or ![]() to make the most
stable cation
to make the most
stable cation
PP 9-03 Answer:
PP 9-04 Answer: C > A > B > D
PP 9-05 Predict the organic products of the following nucleophilic substitution reactions, all of which are carried out in polar aprotic solvent. Show stereochemistry at chiral carbons. Hints: Na2CO3, sodium carbonate, is a weak base. For part (f): What is the conjugate acid of NH2-? What is the pKa of this conjugate acid, and what is the pKa of a terminal alkyne?
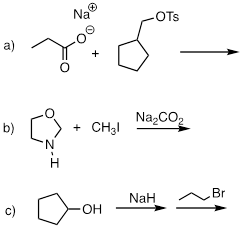
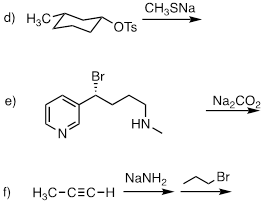
PP 9-06 Which of the reactions in the previous problem has a unimolecular rate determining step? Explain.
The only possible SN1
reaction is e). There is a good leaving group (Br(-)). The hypothetical
carbocation is π-stabilized (benzylic), and the base CO2(-2) is
not strong enough to evolve a strong nucleophile form the amine. These three
arguments favor SN1 mechanism. If stereochemistry is inverted
cleanly, see hypothetical SN2 product above, then the mechanism is SN2.
To the extent that optically pure reactant gives rise to racemic product then
the mechanism is pure SN1 or a mix between SN1 and SN2.
PP 9-07 From the following pairs, select the compound that would react more rapidly with bromomethane in acetone solvent.
a) water or hydroxide ion
b) CH3S- or CH3OH
c) CH2S- or CH3SH
d) acetate ion or hydroxide ion
e) diethyl sulfide or diethyl ether
f) dimethylamine or diethylether
g) trimethylamine or 2,2-dimethylpropane
Look for the best nucleophile. Look
for reactive lone pairs.
PP 9-08 Methyl iodide (0.10 mole) is added to a solution that
contains 0.10 mole NaOCH3 and 0.10 mole NaSCH3.
a) Predict the
most abundant neutral organic product that would form, and explain your
reasoning.
b) Assume that
you isolate a mixture the major product (which you predicted in part) along
with a smaller amount of a different nucleophilic substitution product. Explain
briefly but specifically how you could use 1H NMR to determine the
ratio of the two products in the mixture.
PP 9-09 For each pair of compounds, predict which will more rapidly undergo solvolysis in methanol solution.
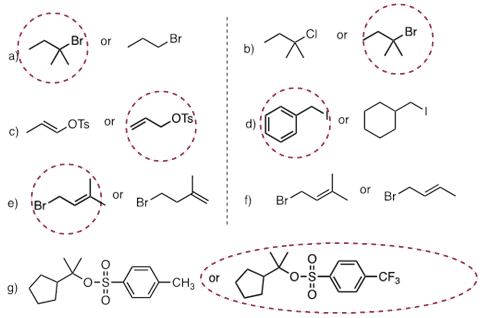
PP 9-10 Predict the solvolysis product(s) of each of the reactions below. Consider both regiochemistry and stereochemistry.
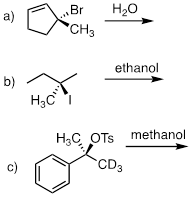

e) Draw a complete curved-arrow mechanism for the formation of the secondary allylilc alcohol product in part (a).
![]()
PP 9-11 Show
starting compounds that would lead to the following products through nucleophilic
substitution reactions.
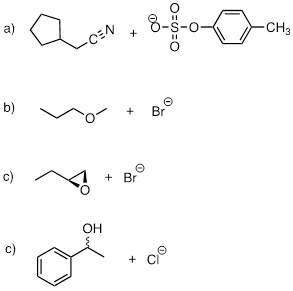
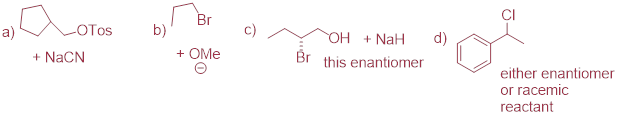
PP 9-12 The fused ring compound shown below is very unreactive to nucleophilic substitution, even with a powerful nucleophile. Explain. (Hint: consider bond geometry - a model will be very helpful!)
![]()
Nucleophiles
cannot access σ* doe to the ring behind the C─Cl
bond.
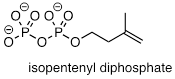
PP 9-13 Laboratory
synthesis of isopentenyl diphosphate - the 'building block' molecule used by
nature for the construction of isoprenoid molecules (section 1.3A) - was
accomplished by first converting isopentenyl alcohol into an alkyl tosylate then displacing the tosylate
group with an inorganic pyrophosphate nucleophile. Based on this verbal
description, draw a mechanism for the second (nucleophilic substitution) step,
showing starting and ending compounds for the step and curved arrows for
electron movement. 51, 4768).
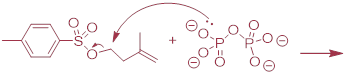 product shown above
product shown above
PP 9-14 Choline, an important neurotransmitter in the nervous system, is formed from 2-(N,N-dimethylamino)ethanol:
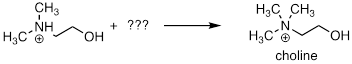
a) Besides the enzyme and the starting compound, what other important biomolecule do you expect plays a part in the reaction?
SAM see above
b) Draw a mechanism for the reaction.
See above.
Use the mechanism in Exercise 9.9.2 except 2-(N,N-dimethylamino)ethanol
is the nucleophile.
c) Briefly explain how 1H NMR could be used to distinguish between the substrate and the product of this reaction.
In
protonated 2-(N,N-dimethylamino)ethanol there will be
a singlet for the CH3 groups that integrates to 6: 2: 2 vs. the two
CH2 groups. In the product choline there will be a singlet for the
CH3 groups that integrates to 9: 2: 2 vs. the two CH2
groups.
PP 9-15 The
following is a reaction in the biosynthesis of morphine in opium poppies. (Science 1967, 155, 170; J. Biol. Chem 1995, 270,
31091). 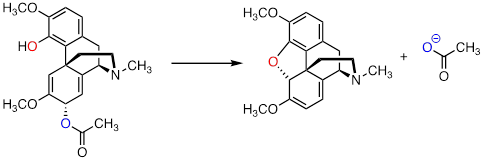
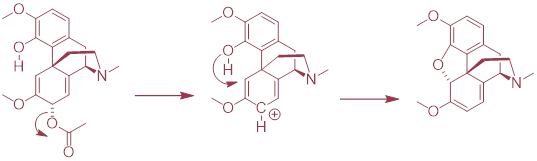
a) Draw a complete mechanism, assuming an SN1 pathway.
b) What would you expect to be the most noticeable difference between the IR spectrum of the product and that of the substrate?
The hydrogen bonding in the OH
stretching region disappears ~3500 cm─1. The carbonyl of the
ester disappears at 1700 cm─1.
c) This reaction is an example of the regiospecificity of enzymatic nucleophilic substitution reactions noted earlier in the chapter. Draw two alternate nucleophilic, ring-closing steps for this reaction (leading to different products from what is shown above), and explain why these alternate pathways are both less favorable than the actual reaction catalyzed by the enzyme.
All other ring closures are
strained. This is best shown by building a model.
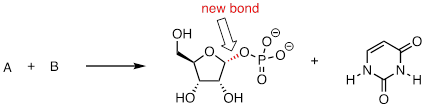
PP 9-16 The enzymatic reaction below, which is part of the metabolism of nucleic acids, proceeds by an SN1 mechanism. The new bond formed in the substitution is indicated.
a) Predict the structures of the two substrates A and B.
The
nucleophile is PO4(-). The electrophile is:
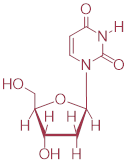
b) Draw a complete mechanism, and use resonance drawings to illustrate how both the carbocation intermediate and the leaving group are stabilized.
The lone
pair on the O atom stabilizes the cation with conjugation. ─(+)O=C─
The
leaving group is stabilized by the fact that the liberated lone pair is sp2
hybridized at the relatively electronegative N atom.
PP 9-17 Below is the first step of the reaction catalyzed by anthranilate synthase, an enzyme involved in biosynthesis of the amino acid tryptophan.
a) This reaction is somewhat unusual in that the leaving group is a hydroxide anion, which is of course is normally thought to be a very poor leaving group. However, studies show that an Mg+2 ion is bound in the active site close to the hydroxide. Explain how the presence of the magnesium ion contributes to the viability of hydroxide as a leaving group.
b) Draw a complete mechanism for the reaction, assuming an SN1 pathway.
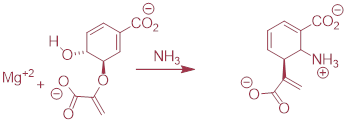
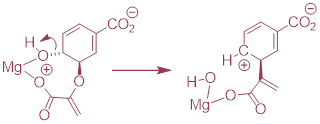
PP 9-18 The reaction below is part of the biosynthesis of
peptidoglycan, a major component of bacterial cell walls. Is it likely to
proceed by a nucleophilic substitution mechanism? Explain.
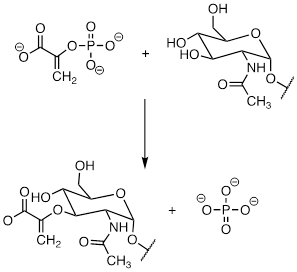
No, because the O atom that is
displaced is connected to a C atom by an sp2 hybridized bond at C.
Due to σ sp2 being so low in energy (so stable), the σ*sp2
orbital is higher energy than the π* orbital, so the π* orbital is
likely the LUMO in this reaction.
PP 9-19 Compare the reaction below, catalyzed by the enzyme AMP-DMAPP transferase, to the protein prenyltransferase reaction we learned about in section “9.9.3 A Biochemical SN1/SN2 Hybrid Reaction”, the mechanism of which, as we discussed, is thought to be mostly SN2-like with some SN1-like character.
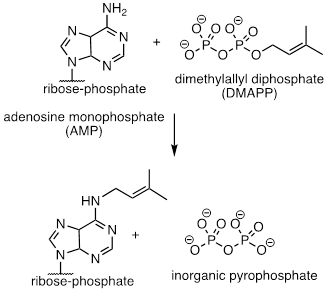
a) Is the AMP-DMAPP transferase reaction below likely to have more or less SN1 character compared to the protein prenyltransferase reaction? Explain.
The
reaction above is likely to have less SN1 character. The cation is
not as stable above as it is in the prenyl transfer. Prenyl is more π-bound (conjugated) than
dimethylallyl.
PP 9-20 In a classic experiment in physical organic chemistry, (R)-2-iodooctane was allowed to react (non-enzymatically) with a radioactive isotope of iodide ion, and the researchers monitored how fast the radioactive iodide was incorporated into the alkane (the rate constant of incorporation, ki) and also how fast optical activity was lost (the rate constant of racemization, kr). They found that the rate of racemization was, within experimental error, equal to twice the rate of incorporation. Discuss the significance of this result - what does it say about the actual mechanism of the reaction?
The rate determining step is the loss of I(-) to produce the achiral cation. The mechanism must be wholly SN1.